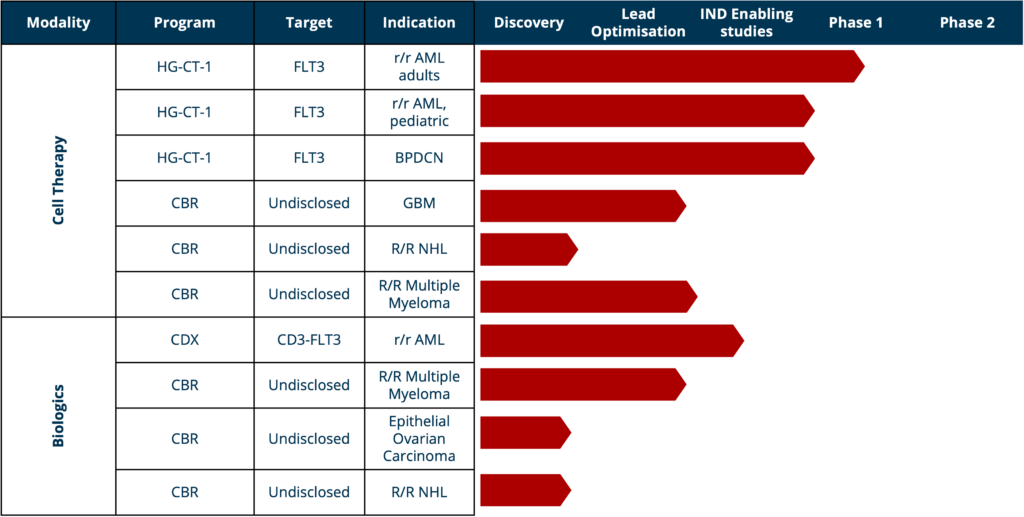
Pipeline
Blood cancers affect over 1.1 million people in the United States annually, with over 156,000 new cases estimated in 2014 alone.
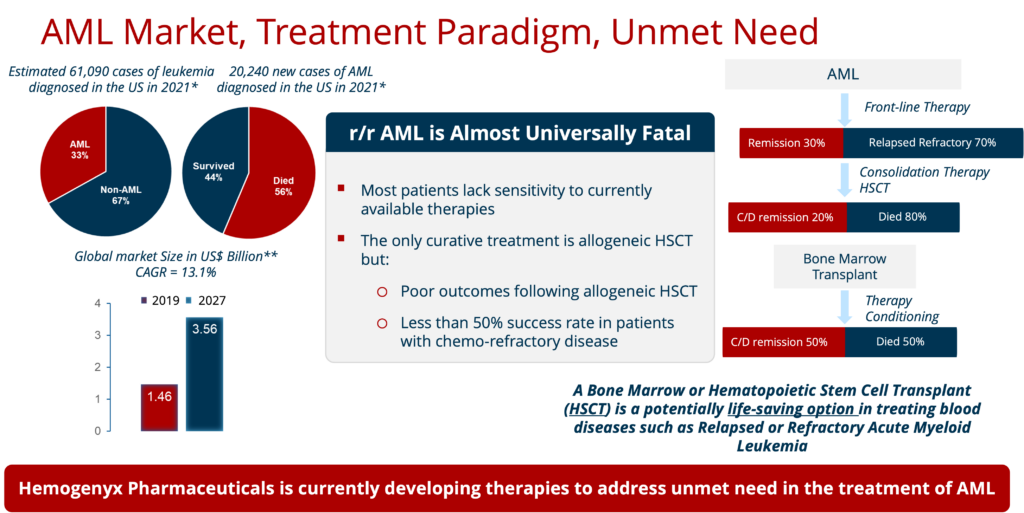

- The only curative treatment is an allogeneic HSCT with less than a 50% success rate in patients with chemo-refractory disease
- Most patients lack sensitivity to currently available therapies
- Poor outcomes following allogeneic HSCT

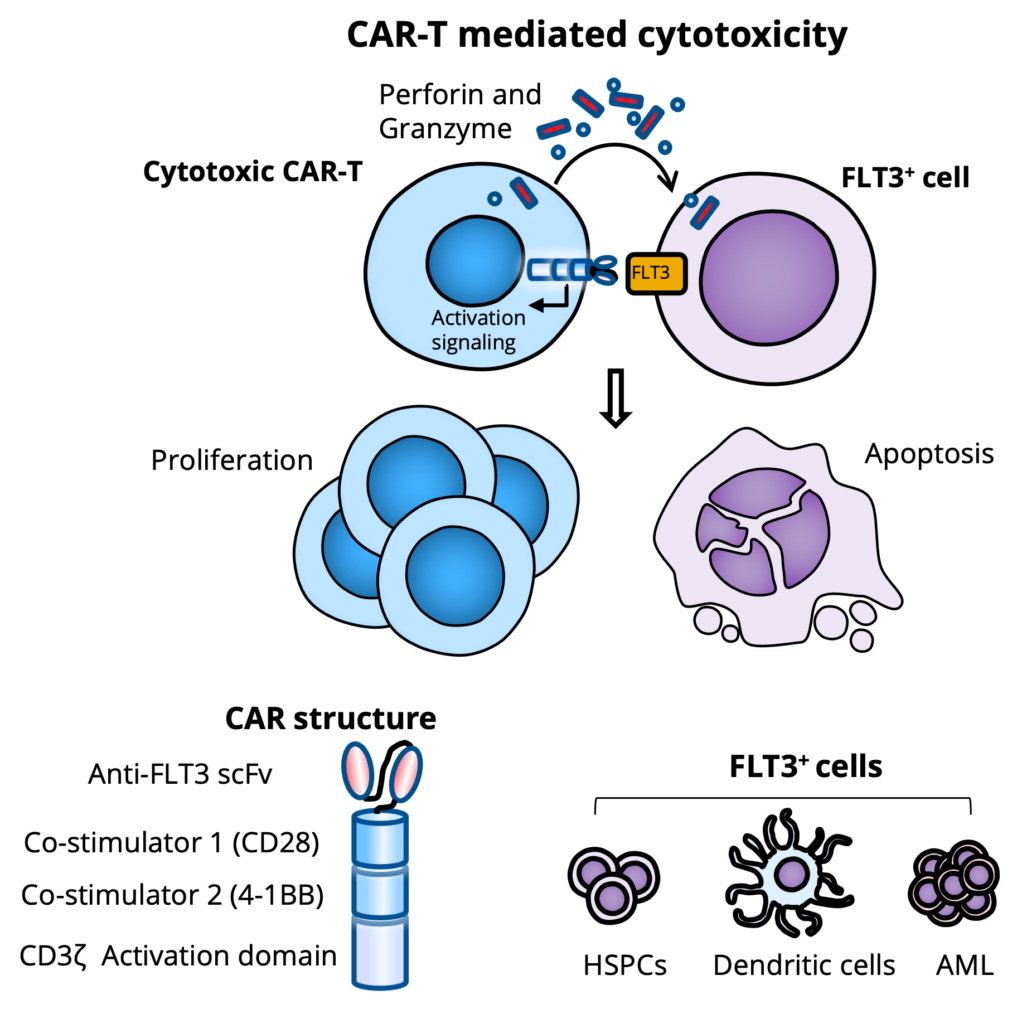
- A novel third-generation HEMO-CAR-T (Anti-FLT3 CAR-T cells) to eliminate malignancy in patients with FLT3+ R/R AML
- A novel bispecific monoclonal antibody CDX (FLT3-CD3) to eliminate malignancy in patients with FLT3+ R/R AML and potentially condition bone marrow transplant
After exhausting all options, including chemotherapy, radiation therapy and immunotherapy, Hemogenyx Pharmaceuticals seeks to tackle the dangers and limitations associated with the current standard of care with its revolutionary technology.
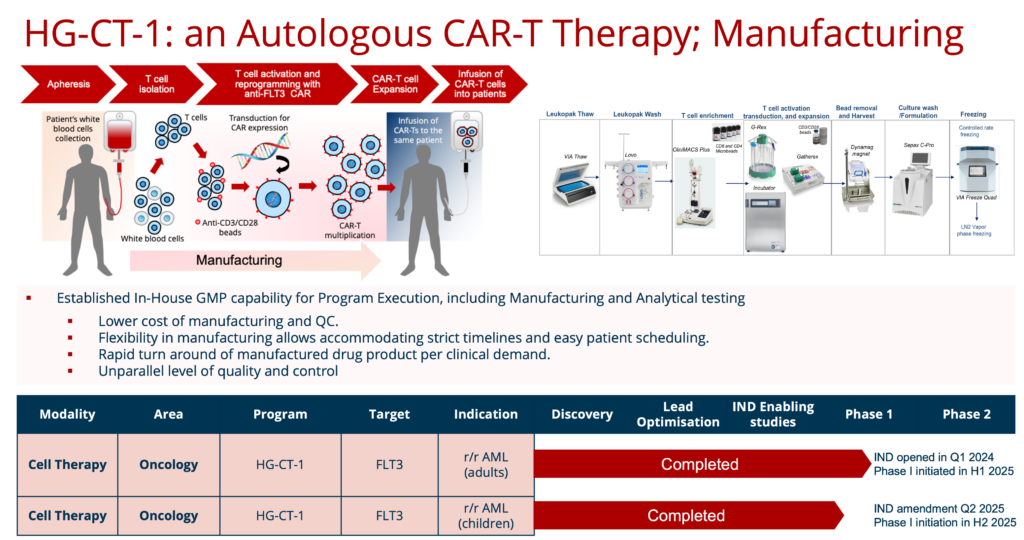
HG-CT-1 (CAR-T)
Autologous 3rd generation CAR-T therapies
Mechanism of Action: cytotoxic lymphocyte (CTL)-mediated cytolysis
- Clinically proven safety
- Established Freedom to Operate
HG-CT-1
- Targeting FMS-like tyrosine kinase 3 receptor (FLT3), which is highly expressed by AML blasts in a majority of patients
- Improved vector design for safety
- Proprietary anti-FLT3 humanized antibody (scFv)
- No off-target binding other than FLT3
- No FLT3 Ligand (FLT3L) competition, avoiding possible reduction of HG-CT-1 efficacy
- Proven in vitro and in vivo antitumor activity
- Phase 1: two patients dosed, third recruited – MD Anderson
CDX
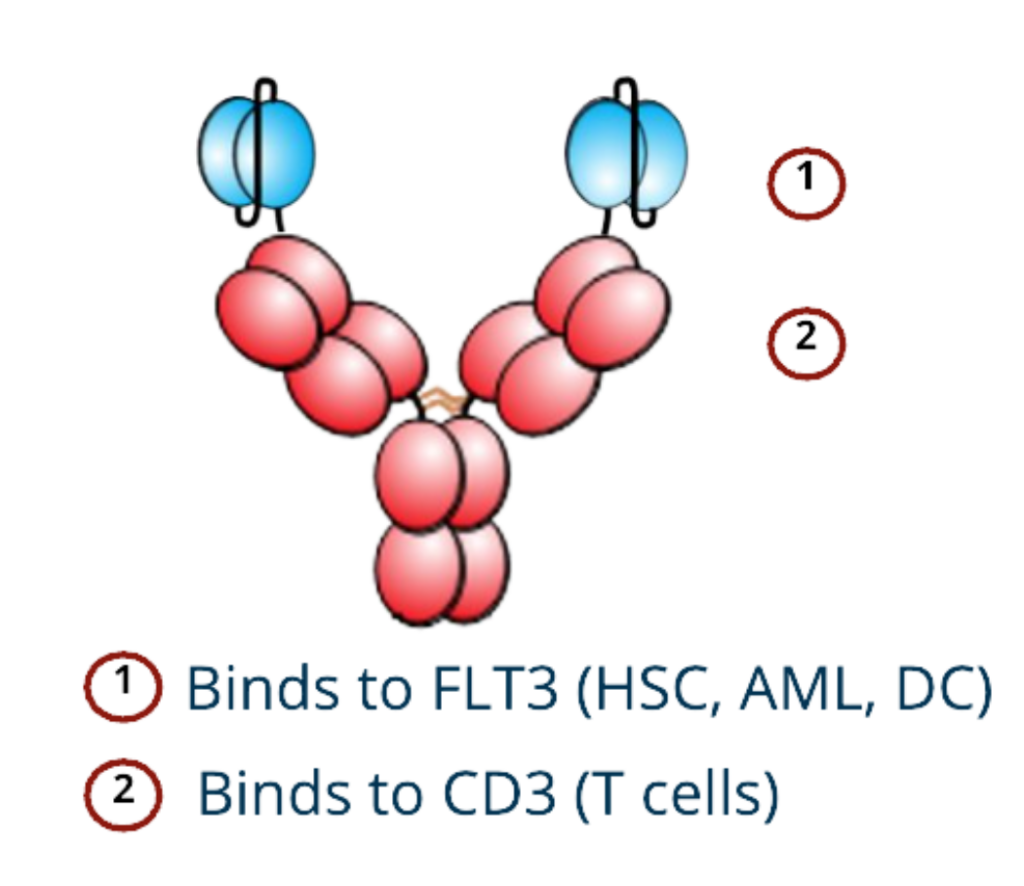
Novelized Humanized FLT3-CD3 Bispecific Antibody
- An “off the shelf” (non patient-specific) product is highly expressed by AML blasts in a majority of patients
- Eliminates AML-derived cells transplanted into humanized mice
- High affinity binding to FLT3
- No FLT3 Ligand (FLT3L) competition
- Unique bi-specific structure: bi-valent FLT3 and bi-valent CD3 binding
- Highly Potent and allows to target low-FLT3 expressing cells of different sizes
- Designed to minimize potentially dangerous non-specific T-cell activation
- Cross-reacts with Rhesus monkeys that will be used for further in vivo testing
- Functional synergy with epigenetic modifying drugs, BET inhibitors and checkpoint inhibitors or conditioning regimens for HSCT
- Exclusively licensed global rights and developed in collaboration with Eli Lilly
Efficacy – Anti-FLT3 ‘arm’ of CDX does not compete with the FLT3 Ligand expressed by a variety of cell types on their surface including AML, avoiding possible reduction of the antibody efficacy since no competition with endogenous ligand
Safety – CDX does not activate T cells in the absence of target cells.
Path to IND:
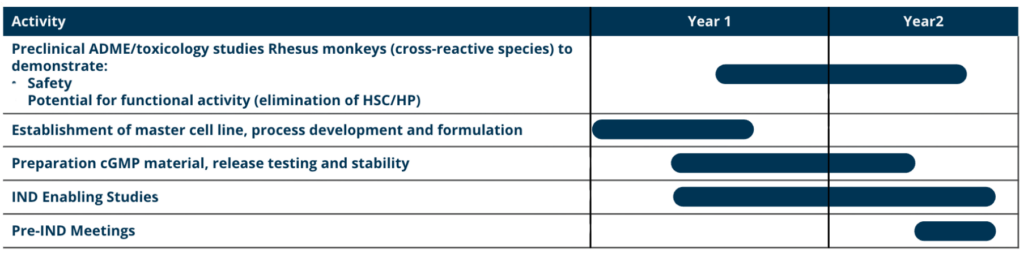
Clinical Plan:
- Initial clinical study to be conducted in relapsed or refractory FLT3+ AML and ALL patients pre-qualified for HSC/HP transplantation to obtain preliminary data on safety (dose escalation), tolerability and Ph II dose (expected initiation in 2025/2026)
- The study will be expanded into pediatric R/R AML and KMT2A rearranged acute lymphoblastic leukemia (ALL)
- Potential upside for early signal of activity demonstrated as:
- Elimination of malignant cells (FLT3+ AML)
- Elimination of HSC/HP (myeloablative conditioning)