Technology
HEMO-CAR-T Immunotherapy
Hemogenyx Pharmaceuticals has constructed Chimeric Antigen Receptor (CAR) programmed T cells, termed HEMO-CAR-T, for the potential treatment of Acute Myeloid Leukemia (AML). HEMO-CAR-T is made using Hemogenyx’s proprietary humanised monoclonal antibody against a target on the surface of AML cells.
This cutting-edge application of cell-based immune therapy represents a further product candidate for Hemogenyx Pharmaceuticals in a new and exciting area of the treatment of blood cancers, and a potentially more benign and effective form of therapy that, if successful, would have a major impact on treatment and survival rates.
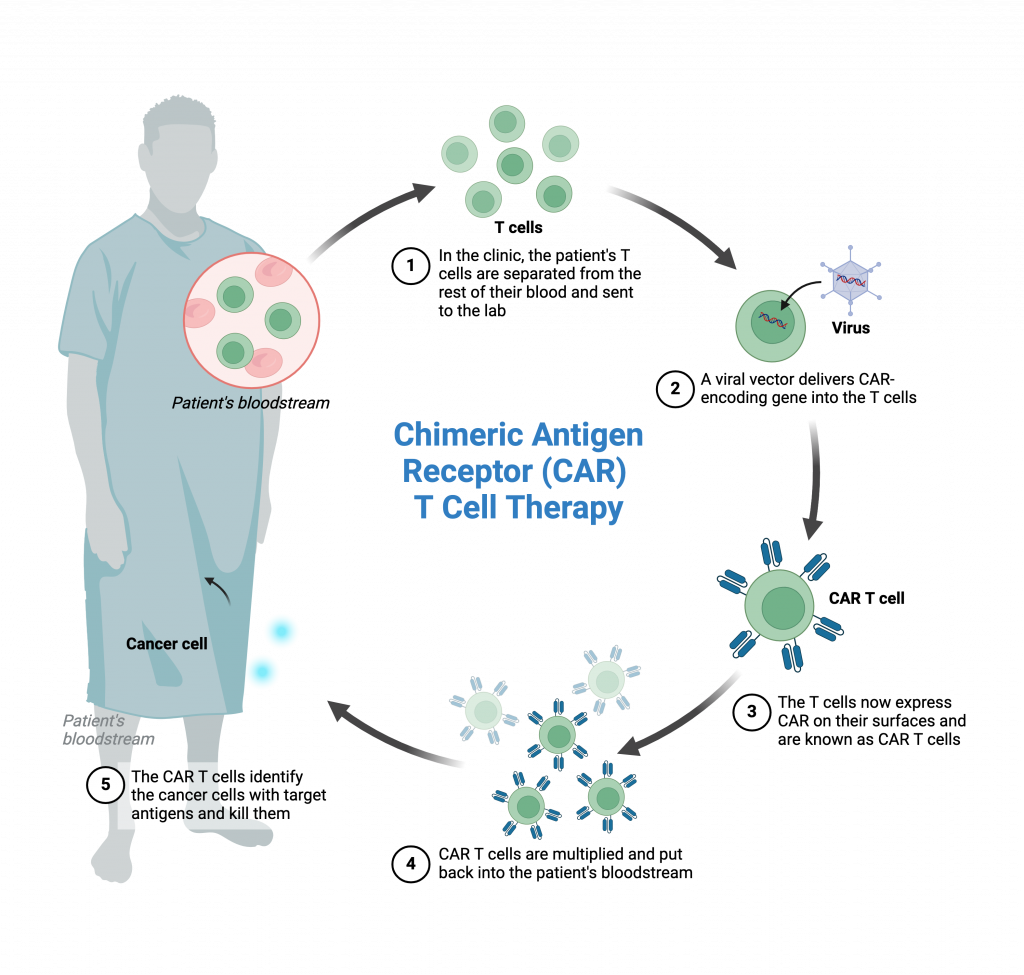
CAR-T therapy is a treatment in which a patient’s own T cells, a type of immune cell, are modified to recognise and kill the patient’s cancer cells. The procedure involves:
- Isolating T cells from the patient
- Modifying the isolated T cells in a laboratory using a CAR gene construct which allows the cells to recognise the patient’s cancer
- Amplifying (growing to large numbers) the newly modified cells
- Re-introducing the cells back into the patient
Hemogenyx Pharmaceuticals has successfully demonstrated that HEMO-CAR-T is able to programme human T cells, that is, to convert them into HEMO-CAR-T, so that they identify and destroy human AML-derived cells in vitro.
CDX for AML
What is it?
CDX bi-specific antibody for the treatment of relapsed/refractory. AML and conditioning of bone marrow transplantation. Phase I is projected to start in 2025-2026. Developed in collaboration with Eli Lilly & Co. Exclusive world-wide license. In order to treat acute amyloid leukemia (AML) and to obviate the use of chemotherapeutic agents for conditioning of patients undergoing BM/HSC transplantation, Hemogenyx Pharmaceuticals developed a method of selective targeting and elimination of FLT3+ cells (AML and hematopoietic stem cells/hematopoietic progenitors (HSC/HP) in patients using highly specific CDX antibodies.
CDX antibodies belong to a class of bi-specific antibodies that redirect patients’ own immune cells to eliminate AML cells and HSC. In sum, CDX antibodies have the potential to both eliminate malignant leukemic cells and increase the efficiency of conditioning while diminishing the side effects that accompany traditional methods of patient conditioning.
CBR for Cancer and Infectious Disease
A novel paradigm for targeting Cancer, Neurodegenerative disease treatments and creating Antivirals
Mechanism of Action: Programming or redirection of myeloid immune cells such as macrophages using novel synthetic proteins
Chimeric Bait Receptor Platform (CBR) is an immunotherapy that will be utilized to prevent and combat infection by any known or emerging virus. CBR will also be used to program innate immunity to combat certain types of cancer
Expected Significant Advantages:
As CBR-programmed macrophages:
- Penetrate solid tumors
- Modulate solid tumor microenvironment for better efficacy
- Better safety profile than standard-of-care treatments
- Immunize host against targeted malignant cells
As Antivirals:
- Single therapeutic targeting multiple viral infections
- Long shelf life at ambient temperature
- Easy deployment/administration at ambient temperature


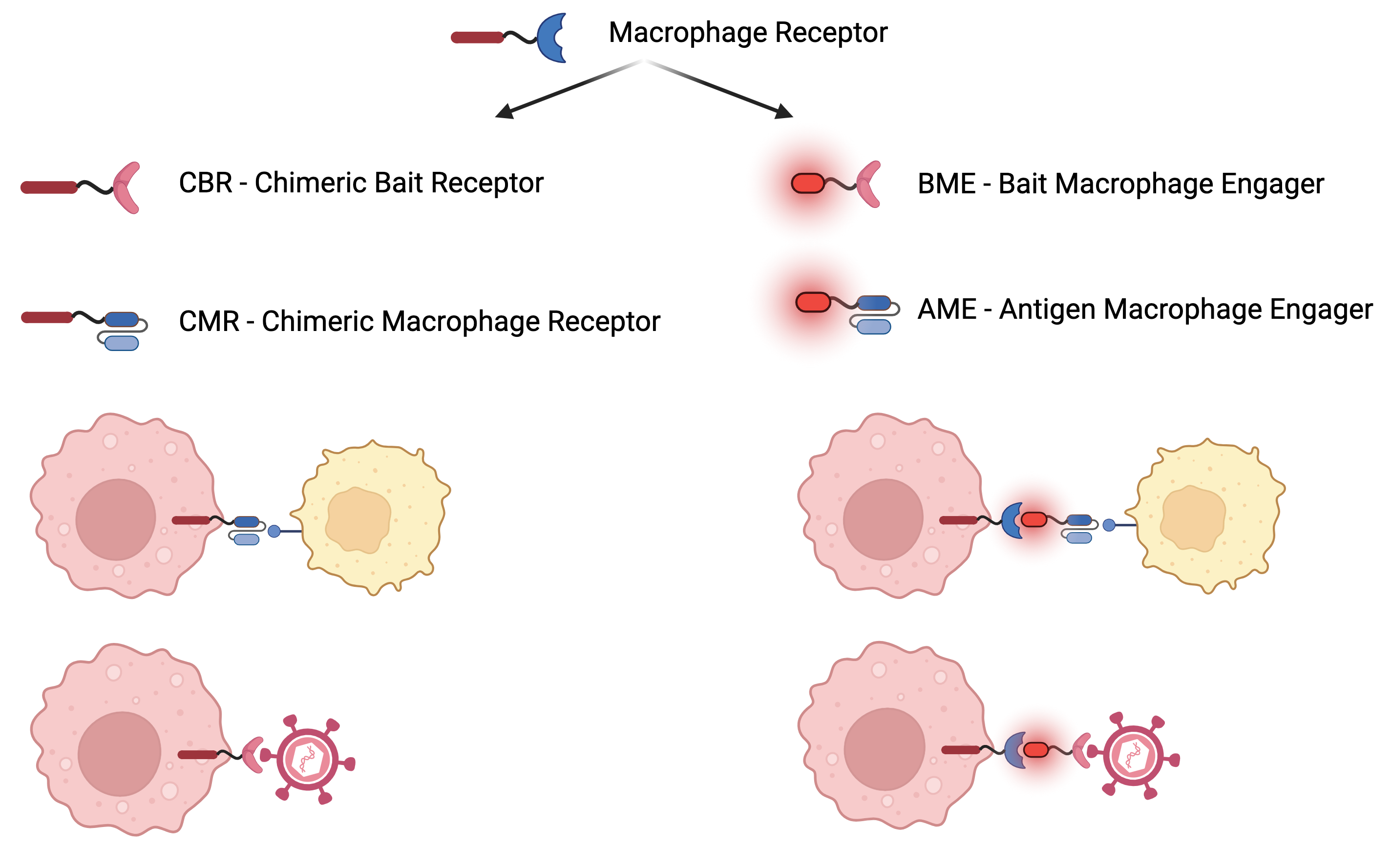
Exclusive IP for CBR, proof of concept (“POC”) is achieved for SARS-COV-2 (COVID19). A possibility to develop a treatment to combat multiple viruses that belong to different viral families. The multi-billion market for viral infections and certain types of cancers. It is being expanded into solid tumors and neurodegenerative diseases.